Glass Packaging Case Study - Rethinking the pharma packaging paradigm: A case for molded glass
Language: English Download - (0.97 Mo)Velocity Vials
IDENCY, the next generation of glass vials
SGD Pharma - PROSEAL+: SGD Pharma’s pre-treated glass for thermosealing applications
Why choose Type I molded glass solutions?
SGD Pharma - Worried about glass lamellae formation in injectable drugs
Are you at risk of glass vial shortages ? Turned to SGD Pharma for support
AXpert
AXecure
AXess
Sterinity - High quality Ready-To-Use sterile vials - 100ml - range extension
Need to speed up your time-to-market for parenteral drugs ?
SGD Pharma - EasyLyo - Simply stronger molded glass vials
Ensiemo, your partner for a safe, qualitative & compliant packaging solution
SGD Pharma Clareo - Type II Premium Injection Vials
SGD Pharma - Siliconization process
Looking for the latest info on global CBD regulations ?
Latest global regulatory updates in the fast-moving CBD industry
Does your pharma packaging meet child resistant requirements ?
SGD Pharma Clareo - инъекционные флаконы типа II Premium
SGD Pharma Clareo - Tipo II Frasco Injetàvel Premium
SGD Pharma embossed marks on glass vials
Sterinity - High quality Ready-To-Use molded glass vials - Range extension
SGD Pharma - CSR report 2022
Protecting children with lifesaving packaging
Welcome to SGD Pharma
Why partner with SGD Pharma?
Explore the world of SGD Pharma packaging - Part 1
Explore the world of SGD Pharma packaging - Part 2
SGD Pharma - Non-conformities App
Bienvenue chez SGD Pharma
Bienvenidos a SGD Pharma
SGD Pharma - 2021 Corporate Social Responsibility Report
Parlons verre !
Des métiers en images - Episode 1 - Conducteur mécanicien bout chaud - SGD Pharma x La Glass Vallée
Des métiers en images - Episode 2 - Mécanicien IS - SGD Pharma x La Glass Vallée
Des métiers en images - Episode 3 - Contrôleur verre bout froid - SGD Pharma x La Glass Vallée
Aptar Interview: A Conversation With Carole Grassi Mircich
#LiveWithChaudhrey with Carole Grassi Mircich, SGD Pharma, at #CPHIWW 2021 Milano
#LiveWithCHaudhrey Carole Grassi Mircich SGD Pharma at PharmaPack 2020 molded glass vials.
#ChatsWithChaudhrey with Carole Grassi Mircich, SGD Pharma, on the new Moulded Glass Vials Offering
#ChatsWithChaudhrey with Najet Mubarki Senior Product Manager SGD Pharma
#ChatsWithChaudhrey #2020Positives & Learnings #2021Predictions with SGD Pharma
Interview with SGD Pharma - Pharma's Almanac - Road to the Future
Why pharma packaging choice matters in protecting children's lifes
Alternative vial design to reduce breakage risk during lyophilization
Multiple glass vials sourcing to secure your parenteral drug supply.
Press release
SGD Pharma showcases Sustainable Glass Innovation and Decorative Excellence at Paris Packaging Week – PCD 2026
Language: English Download - (0 Mo)SGD Pharma leads pharma packaging innovation and decarbonization at Pharmapack Europe 2026
Language: English Download - (0 Mo)SGD Pharma Zhanjiang Becomes China’s First Glass Manufacturer to Achieve ISO 14021 Certification for PCR Packaging
Select your language:SGD Pharma presents new innovations at CPHI Frankfurt 2025
Select your language:Corning and SGD Pharma Inaugurate Glass Tubing Facility in Telangana To Expand Pharmaceutical Packaging Manufacturing
Language: English Download - (0 Mo)A Strategic Step Forward: Alphial S.r.l. Joins SGD Pharma
Select your language:SGD Pharma releases new sustainability report outlining industry-leading pharmaceutical glass CSR performance
Language: English Download - (0 Mo)SGD Pharma showcases its SEALIAN platform at Pharmapack Europe 2025
Language: English Download - (0 Mo)SGD Pharma spotlights new optimized solutions at CPHI worldwide 2024, continuing its leadership in sustainability and innovation
Language: English Download - (0 Mo)SGD Pharma showcases new post-consumer recycled glass packaging at Luxe Pack Monaco 2024
Language: English Download - (0 Mo)SGD Pharma held its European Suppliers Day bringing together about 100 stakeholders of the glass and sustainability ecosystem on June 18th
Select your language:SGD Pharma releases its latest sustainability report underscoring its global commitment to the highest environmental, social and governance standards
Language: English Download - (0 Mo)SGD Pharma invests €20M to build furnace and upgrade industrial facility at its Zhanjiang plant in China, advancing its decarbonisation roadmap
Language: English Download - (0 Mo)SGD Pharma announces the appointment of Philippe Pourquery as Group Financial Officer and member of the Executive Committee
Select your language:SGD Pharma successfully trials hydrogen burners at its Saint-Quentin-Lamotte (SQLM) plant to accelerate its ambitious decarbonization roadmap
Language: English Download - (0 Mo)SGD Pharma awarded 2024 gold EcoVadis rating in recognition of its commitment to sustainability
Language: English Download - (0 Mo)SGD Pharma optimizes global fill and finish operations with the introduction of its new range of Type I tubular vials featuring Corning’s Velocity® Vial coating technology for enhanced product quality and patient safety
Language: English Download - (0 Mo)SGD Pharma expands capacity of siliconized glass vials with the announcement of a new siliconization line at its Center of Excellence for Type I glass in France
Language: English Download - (0 Mo)SGD Pharma announces the appointment of Fabio Invernizzi, General Manager West Business Unit
Language: English Download - (0 Mo)SGD Pharma defines 2024 growth strategy as it strives for market leadership with investment achievements
Language: English Download - (0 Mo)EUROPEAN CONTAINER GLASS FEDERATION NEW LEADERSHIP TEAM APPOINTED AT FLACONNAGE BOARD OF DIRECTORS
Language: English Download - (0 Mo)SGD Pharma unveils NOVA lightweight glass bottle packaging innovation, addressing the commitment to environmental responsibility from cosmetic and beauty brands Paris
Language: English Download - (0 Mo)SGD Pharma commits to near-term Science Based Targets initiative as part of sustainability drive
Language: English Download - (0 Mo)SGD Pharma to present its high-end glass packaging innovations tailored to the cosmetics and beauty industry at Luxe Pack Monaco 2023
Language: English Download - (0 Mo)SGD Pharma’s latest annual sustainability report details global environmental, social and ethical efforts
Language: English Download - (0 Mo)Corning and SGD Pharma announce joint venture to open new glass tubing facility and expand access to Corning® Velocity® Vial technology in India
Language: English Download - (0 Mo)Continued focus on innovation to be presented by SGD Pharma at Pharmapack Europe 2023
Language: English Download - (0.15 Mo)Committed to staying ahead with pioneering glass packaging solutions that answer the changing needs of the pharmaceutical industry, SGD Pharma is set to showcase its latest innovations at CPhI worldwide 2022
Language: English Download - (0 Mo)SGD Pharma returns to Luxe Pack Monaco with new proposition for premium glass packaging for cosmetics and beauty
Language: English Download - (0.14 Mo)SGD Pharma announces new CEO as it targets further growth
Select your language:SGD Pharma expands cosmetics glass packaging offering, appoints industry leader
Language: English Download - (0.15 Mo)SGD Pharma launches industry first Ready-to-Use sterile 100 ml molded glass vials in SG® EZ-fill® packaging technology
Select your language:2022 Année Internationale du Verre, une double cérémonie d’ouverture à l’ONU à Genève et au Palais du Luxembourg à Paris
Language: French Download - (3.74 Mo)SGD Pharma speeds up time to market with industry first Ready-to-Use SG EZ-fill® Nest & Tub packaging solution for molded glass vials for parenteral drugs
Select your language:Key milestone in molded glass: SGD Pharma elevates Type I offering with three tailored product and service solutions
Select your language:SGD Pharma takes lead in sustainability, awarded Platinum EcoVadis rating for the first time
Select your language:SGD Pharma continues customer-led innovation with range extension for Sterinity Ready-to-Use molded glass vials: adds amber 50ml size ISO and EasyLyo in Tray
Select your language:Q&A
- All topics
- Continuous improvements to Type 1 molded glass
- COVID-19
- General
- Investment & future-proofing
- Vial manufacture
Operation Marketing & Communication Manager |
Alice ACHACHE
Media partner | The Scott Partnership
Yes. Our state-of-the-art manufacturing plant in Vemula near Hyderabad in India, completed in 2013, is registered to manufacture Type I glass compliant with Japan, European, India and US pharmacopeias. The best-in-class tubular vials and ampoules manufactured at Vemula are used in India for COVID-19 vaccine vial packaging. As well as its local and national supply of tubular vials, the Vemula site also has a major output of Type I molded glass for the Asian, EMEA and USA markets and is used as part of the global supply chain for many pharmaceutical companies.
We offer a broad range of sizes and finishes in Type I, II, or III, amber and clear glass, as well as innovative added-value services like internal siliconization or protective plastic coatings.
We build long-term relationships with our customers and are responsive, reliable, transparent and highly collaborative. Working closely with customers and researching the latest technologies, we seek specific challenges to address through the research or application of new technology solutions.
Our SQLM site in Normandy, France, has a new operations facility opened in 2013 and the Vemula state-of- the-art plant in India was completed in February 2018, with new furnaces, each with ISO 8 cleanrooms (1700m 2 and 2400 m 2 ) Together these plants offer 31,800m 2 and 11,000m 2 , yielding a production capacity of 1.5 million vials and ampoules daily from SQLM and 1 million vials a day from Vemula. This, together with our ex-stock capacity, allows us to meet the vaccine demand while still servicing our other customers.
During 2020, SGD Pharma continued its daily operations and even improved performance in all plants, strengthened clients’ trust and gained market shares, accelerated R&D capabilities, and recruited and improved training efforts. Our plants in Asia and Europe are all subject to the highest level of safety measures to ensure the health and safety of employees while continuing manufacturing operations.
To manufacture and ship vaccines globally requires highly managed logistics and access to continuous and secured supply of ancillary materials to ensure vaccine distribution does not face any bottlenecks. As a glass primary packaging company, we are working tirelessly to help guarantee this access for global COVID-19 immunization campaigns.
To help prepare this vaccine for worldwide distribution, SGD Pharma is offering access to our entire manufacturing output from our state-of-the-art ISO 15378 certified plant in Normandy, France, where our daily capacity stands at 400 to 500Ku of internationally recognized Type I neutral molded glass vials, available in many sizes including 10ml, 20ml, 30ml and 50ml.
Since 1896: we draw on more than 100 years of expertise inherited from Saint-Gobain, and are renowned worldwide as a technical leader in glass packaging.
We employ over 3,000 experts in R&D, Sales, Marketing, quality, regulatory, environment, health and safety, and operations. Our teams work together to solve tomorrow’s challenges today.
5 manufacturing plants across 4 countries (France, India, Germany, China). We combine control of the glass process with the implementation of GMPs for pharmaceutical primary packaging in all our manufacturing facilities.
Following the completion of our Vemula plant in 2013 and SQLM in 2016, we plan to continue investing in the latest technology for our facilities, and provide our customers with unwavering product quality. This is demonstrated through our upcoming 2021 product initiatives, which includes elevating our Type I molded glass product portfolio, to provide customers with solutions that meet their individual business and product needs. By making continuous improvements to glass composition, production processes and equipment, and quality criteria, SGD Pharma remains the leader in molded glass packaging.
We aim to improve and protect the health of patients by providing high quality, reliable and innovative primary glass packaging to our pharmaceutical customers.
Two plants are best-in-class facilities dedicated to Type I glass (Saint-Quentin-Lamotte (SQLM) in Northern France and Vemula in India). SQLM is a Center of Excellence for Type I molded glass. Type II glass for anesthetics is produced in Sucy-en-Brie, France and Kipfenberg, Germany. Type III amber glass for analgesics is produced in Sucy-en-Brie. All manufacturing facilities are ISO 15378 certified. Vemula, India is also ISO 14001 and 18001 accredited.
We produce over 8 million vials and bottles per day, across plants in Europe and Asia. More than 2 billion vials are manufactured and sold per year.
Between 2020 and 2025, we will rebuild all our 7 furnaces and upgrade them with new technologies (live monitoring systems, better thermal insulation, increased electric boosting). These improvements will eventually enable us to improve our overall energy efficiency by at least 7% by 2025.
In 2019, we commenced a major plant development project Sucy-en-Brie, France. This Capex, 31 million€ in total, is the biggest SGD Pharma investment since the Saint-Quentin- Lamotte plant construction. The Sucy-en-Brie factory, already ISO 15378 & 50001 certified facility with 8 production lines connected to ISO 8 clean rooms, will join a next generation of pharma glass packaging units.
The project includes the complete rebuilding and upgrading of the furnace 2, the modification of the building itself, the full revamping of the ISO 8 cleanrooms, new-generation IS machines and the installation of the most advanced technologies available for automatic product inspection. Thanks to live monitoring systems, better thermal insulation and increased electric boosting, CO 2 emissions will be reduced by 10%. The re-design of circulation areas will improve furthermore the safety of the plant.
Our Type I molded glass vials are resistant and chemically inert (thanks to their high hydrolytic resistance), making them a relevant solution to complete the Type I tubular glass vials offering and respond to this unprecedented surge in demand. They are ideal both to store and transport vaccines globally.
SGD Pharma is a global leader in glass pharmaceutical packaging, answering to the needs of a diverse range of markets including parenteral, nasal, and oral drugs, as well as beauty and nutrition.
SGD Pharma aims to reduce its CO 2 emissions by 5% per tons of goods produced. This equates to a saving of over 10,000 tons of CO 2 by 2025. We are planning to achieve this via:
Participation in FEVE Furnace of the Future Initiative:
We are one of 20 companies from the glass industry who have joined forces to create, fund and test a pilot furnace project. This will be the world’s first large-scale hybrid oxy-fuel furnace to run on 80% renewable electricity. This will replace current fossil-fuel energy sources and reduce CO 2 emissions by 50%.
- Investment in Environmental Performance:
To demonstrate our energy commitment, we had 3 plants ISO 14001 certified (environmental management) in 2019 (Kipfenberg, Zhanjiang and Vemula) and we plan to certify the remaining plants (Sucy-en-Brie et Saint-Quentin) by the end of 2021.
- Investment in Water consumption:
To reduce our water consumption, we installed closed-loop circuits at all our production sites.
In 2019, we reduced our overall water consumption by 18% in comparison to 2018. As a result, we saved more than 156 000 m3 of water which has mainly been achieved by the outstanding performance of our French plant at Sucy-en-Brie.
- Investment in glass re-use:
Close the Glass Loop is a European bottom-up, collaborative, public-private partnership that aims to increase the quantity and quality of available recycled glass. This initiative follows the European Green Deal framework, which recently changed the objectives for the recycling rate to accelerate the transition toward a more circular economy.
- Two key targets have been set:
- Raise the rate of glass collection to 90% by 2030 (up from 76% in 2019)
- Improve the quality of recycled glass to allow more recycled content to be used in a new production loop, better, together.
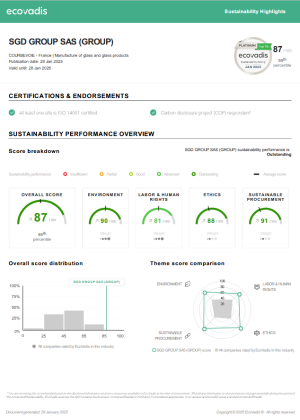
SGD Pharma’s corporate social responsibility (CSR) efforts have been recognized by EcoVadis, an independent evaluator which gave us a Platinum rating in 2020 – placing SGD Pharma in the top 1% of all companies evaluated. This rating demonstrates SGD Pharma’s commitment to sustainable business practice and our alignment with global action towards protecting the environment and tackling social issues. We also received a B score card from CDP, in recognition of our actions towards tackling climate change.
Type I borosilicate glass is used for packaging parenteral drugs. It is the ideal material due to its inertness and chemical stability, which limits interactions with the drug and protects its value as well as patient safety.
The customers of SGD Pharma include global pharma, biopharma, generics and vaccine manufacturers, as well as the cosmetics industry (particularly in China).