New solutions for a vital process: Implementing aseptic filling with Ready-to-Use molded glass vials
Use of the parenteral route of administration has risen sharply in recent years, with parenteral drugs now representing approximately 32% (by volume) of the global market [1]. Parenteral drug delivery, via injection or infusion, is essential for many biologics but is also commonly used for small molecules, low solubility drugs and delivery of nutritional and vitamin therapies. These products are commercialized in both liquid or lyophilized forms, and are available in a broad range of dosages and containers including vials, bottles, ampoules, cartridges, flexible bags and pre-filled syringes. In all cases, aseptic filling of the container is a key step in the manufacturing process with significant investment involved in safeguarding the quality and cleanliness of the primary packaging. There is growing recognition that preparing primary packaging for filling is CAPEX, OPEX and time intensive and that the first steps of the aseptic filling process, though critical to product quality and safety, are not value-added activities.
Rising demand for parenteral drugs comes at a time of intense pressure to reduce costs, across an industry that is seeing a transformation in its structure. Pharmaceutical companies are scaling back on operational ownership with a network of contract research organizations (CROs), contract manufacturing organizations (CMOs) and by aquiring biotech start-ups. A new segment of the market shifts from yesterday’s blockbuster small molecule drugs, requiring large, dedicated-product filling lines, to the manufacture of smaller batches of biotech products in facilities handling multiple products in different container presentations to treat smaller disease populations.
These changes call for agility and a lower asset base, creating an appetite for standard, transferable solutions for aseptic filling to facilitate a focus on core activities of drug development. One notable trend is the growing usage of ready-to-use (RTU) primary packaging for ‘fill & finish’ operations. Due to the success of prefilled syringes, RTU primary packaging has become a preferred option in the aseptic manufacturing process to enhance flexible filling and reduce the Total Cost of Ownership (TCO) to pharma companies. Increasingly, outsourcing the non-core activities of washing and sterilization is becoming common practice for vials and cartridges. RTU options for small volume vials are largely in place but flexible processing solutions for larger sized containers, in the 50 to 250 ml volume range, are not yet fully established; access to larger molded vials in RTU format is critical for key applications.
The introduction of Sterinity by SGD Pharma (Paris, France), a global leader in molded glass pharmaceutical packaging, makes the manufacture of RTU molded glass vials commercially accessible for the first time. Powered by the well-established EZ-Fill® platform from Ompi (Padua, Italy) this new product extends the commercial and practical benefits of RTU to a wider range of applications. In this article we look at what aseptic filling involves, requirements for different types of vials and the drivers of RTU packaging.
Understanding aseptic filling
Parenteral drug products are delivered directly from their packaging to the patient making rigorous preparation of that packaging essential for patient safety. The process of aseptic filling consequently extends from the packaging, through preparation, to filling the drug and securing closure, ready for storage/shipping. A detailed discussion on each of the steps involved lies beyond the scope of this paper but they are described briefly below.
Clean, inspected glassware, as received from a supplier, is subject to washing, sterilization and depyrogenation processes to ensure the removal of:
- Inert particles which present a risk of thrombotic effects for the patient. These may be airborne or arise during glass production and transport.
- Micro-organisms which are endemic within the environment and can trigger serious illnesses such as septicaemia.
- Pyrogens, substances that induce a fever in the patient, a prime example being endotoxins produced by dead bacteria.
There is extensive regulatory guidance in this area, including FDA guidance detailing Current Good Manufacturing Practice (CGMP) [2] and recently updated (2017) EU GMP documentation [3]. As 21 CFR 211.113 makes clear ‘appropriate written procedures designed to prevent microbiological contamination of drug products purporting to be sterile, shall be established and followed. Such procedures shall include validation of all aseptic and sterilization processes.’ Establishing a new aseptic filling line is an extensive and significant undertaking.
Washing/rinsing processes are critical steps in the preparation of glassware for aseptic filling. Used primarily to remove inert particles, they also reduce contamination and endotoxin levels. Using water that meets the same quality standards as that injected into the patient – Water for Injection (WFI) – is crucial. Sterilization processes further reduce micro-organisms, to a defined Sterility Assurance Level, and may include steam heating, dry heating (in an oven or sterilizing tunnel), ethylene oxide treatment, the application of radiation (gamma or electron), filtration and/or other techniques. Depyrogenation is typically a higher temperature treatment than sterilization, carried out for a longer period in a dry oven or (sterilization) tunnel.
The time and money associated with bringing an aseptic line onstream is considerable. Beyond CAPEX for a washer and dry heat sterilizing tunnel, there is substantial ongoing expenditure associated with maintenance, ongoing validation and routine operation. Any manual tasks call for exemplary cleanroom practice, supported by rigorous training. Against this backdrop, switching to RTU can offer significant advantages depending on the level of complexity and customisation required.
Choosing parenteral packaging: Why select molded glass?
Pharmaceutical glass packaging is produced in three glass types as defined by the major pharmacopoeias both USP <660> and EP 3.2.1 specifying performance standards for Type I, Type II and Type III glass containers. Glass containers used for parenteral packaging are differentiated by their chemical composition (Type I, II or III) and with respect to how they are manufactured, with both tubular or molded products in widespread use. The performance of these containers is quantified in terms of:
- Hydrolytic Resistance (a measure of chemical stability)
- Dimensional stability and mechanical resistance
- Cosmetic quality
Identifying the most appropriate packaging is therefore an essential element of product development and crucial for safe and cost-effective manufacture.
Type I is the designation assigned to borosilicate glass, which contains significant amounts of boron oxide (~10%) and has relatively high Hydrolytic Resistance leveml. Hydrolytic Resistance, as measured by pharmacopoeial tests, is quantified in terms of the amount of alkali released by a glass vial filled with ultra pure water, when tested at elevated temperature [4]. High level of Hydrolytic Resistance is associated with low release rates and indicative of high chemical stability. Type I glass also exhibits higher resistance to thermal shock. These attributes make it highly valued within the industry and extremely suitable for parenteral products.
Type III, soda-lime-silica or regular soda lime glass contains significant quantities of both sodium oxide (~15%) and calcium oxide (~10%) and has lower Hydrolytic Resistance than Type I. It can be used for parenteral products if stability testing specifically confirms suitability, with powder applications being the most common. Hydrolytic Resistance can be raised by treating the inner surface of the glass with ammonium sulfate to produce Type II glass which has a similar hydrolytic resistance to Type I but with lower chemical durability.
When it comes to the impact of manufacturing method, molded vials offer high mechanical resistance and low risk of breakage, making them particularly suitable for larger volumes - from around 50 – 3000 ml - and for lyophilized products. Molded products are also often selected for high pH formulations. Tubular vials are most popular in the 1 – 20 ml volume range, offering high cosmetic quality, high dimensional stability and thin walls – a lighter product.
All these factors, and others, affect the packaging chosen for a specific parenteral drug which in turn directly impacts the aseptic filling technology selected. A dedicated manufacturing facility for an established, commercialized drug may handle just one type of vial but CRO, CMO or R&D departments need the flexibility to switch easily between packaging types to assess/handle different volume vials. This requirement for flexibility significantly complicates the development of optimized aseptic filling processes.
A RTU solution
SGD Pharma’s Sterinity offer, through Ompi EZ Fill®, provides an ‘off-the-shelf’ solution for aseptic filling. Sterinity vials are delivered ready for direct introduction into an aseptic fill-finish operation leveraging the advantages of Ompi EZ-fill® secondary packaging, which has been already tested and adopted in a wide range of fill-finish equipment platforms.
Parenteral glass vials are delivered directly to the process area which may be at the pharmaceutical manufacturing site or remote, at a centralized or contract facility serving multiple laboratories and/or production sites. Vials are produced and 100% inspected in a clean room environment at the SGD Pharma plant and then subject to the following processes:
- Washing with WFI followed by drying in a clean room environment
- Depyrogenation in a pharmaceutical grade tunnel.
- Packaging in a nest and tub or tray configuration
- Covering with Tyvek® sheet
- Sealing with a Tyvek® lid followed by steribag bagging (single or double)
- Sterilization by ethylene oxide using a process validated in accordance with ISO 11135 [5].
RTU glass vials which can be then stored with a five year shelf life.
RTU technology decouples the preparatory stages of the aseptic filling process from the final fill and finish process, thereby introducing significant opportunities for outsourcing/process management (see figure 1). Levaring this proven solution eliminates the development, design, construction and validation work associated with a bespoke in-house design accelerating the time required to complete a new project. This is a crucial gain, in both the clinical and industrial settings, whether the goal is a new line or the retrofitting/expansion of existing capacity that can help to speed a product through to commercialization. Furthermore, off-the-shelf designs are rigorously optimized with respect to ease of operation, automation and maintenance. These advantages allow to lower associated OPEX significantly.
These options mean that the TCO of a RTU platform - CAPEX plus OPEX across the lifetime of the unit – are often much lower, than for in-house solutions. This is particularly the case where there is a requirement for combi-lines, aseptic filling lines with the flexibility to handle multiple types of parenteral packaging (cartridges, syringes or vials) without module resetting. This is specially valuable in small batch filling operations.

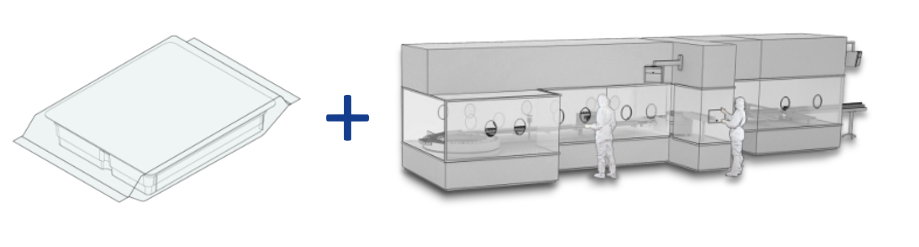
Figure 1: RTU minimizes the assets associated with aseptic filling at a specific site and at the same time can offer significant savings in both CAPEX and OPEX relative to the installation of a bespoke, dedicated system.
Beyond cost saving, RTU offers other practical advantages. Firstly, there is the reassurance of high quality that can easily be replicated, from site-to-site, or by external suppliers if the activity is outsourced. Scale-up is straight forward and issues associated with method transfer are reduced. In certain geographies, using a RTU solution will be economically beneficial even for large scale manufacturing simply because it alleviates quality concerns associated with services such as electricity and water supply including maintenance of the quality standards required for WFI. In addition, the use of RTU offers the flexibility to manage the footprint associated with aseptic filling operations – to minimize the amount of equipment at a certain location, for example - which may be critical for some organizations.
Focusing on SGD Pharma RTU molded glass vials
The Sterinity offer powered by Ompi EZ Fill® uses molded glass vials made from Type I glass of exemplary quality. A steadily expanding portfolio of products in a range of sizes will be commercialized over the next two years, focusing on two core designs: a premium quality ISO design and an optimized EasyLyo® product.
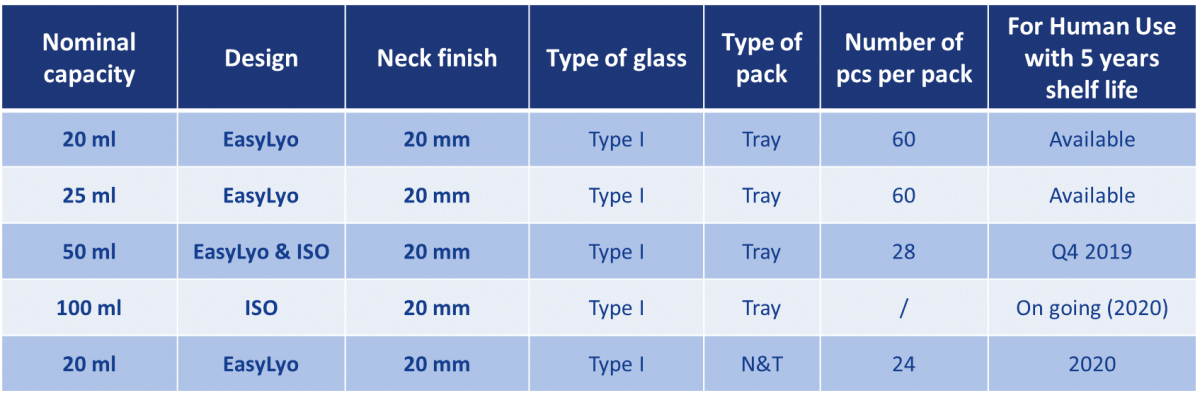
Table 1: The Sterinity platform will extend to a portfolio of molded glass vials answering directly to a wide range of parenteral applications.
The ISO vials provide a high quality molded glass RTU option, compliant to ISO 8362-4. The EasyLyo product is a new generation of molded glass vials that combines chemical and physical strength with superior cosmetic quality, relative to conventional molded alternatives. The external dimensions of these vials are equivalent to tubular designs of the same volume while vial weight is around 30% lower than for standard molded alternatives. Essentially, they combine the strength and resistance to breakage associated with molded vials with the superior packaging/transport characteristics of a tubular product, advantages that are particularly beneficial for lyophilization applications. An optimised bottom to aid heat transfer and an ISO 20 mm neck finish for secured stoppering answer directly to lyophilization requirements.
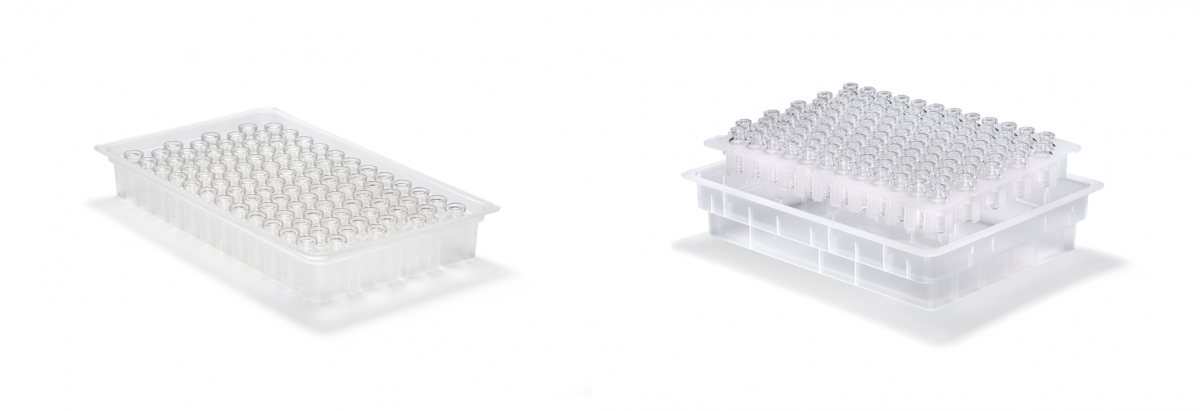
Figure 2: Trays (left) may be the optimal configuration for certain applications but nest and tub (right) offers the flexibility for a single unit to support multiple filling machines using different packaging types.
Both types of vials are currently available for use in tray format; nest and tub configurations are currently in development (see figure 2). In a tray format RTU vials are packaged upside down in trays with dimensions according to Ompi Ez-fill/R secondary packaging, specified for use with defined filling technology. This configuration offer the benefit of maximizing the number of vials in a tray increasing packaging density thus optimizing the throughput of fill and finish operations. Nest and tub is homogenized between all vial formats and with syringes and cartridges to allow adoption of the same handling points in combi lines. Both tray, nest & tub configurations are designed to completely avoid glass-to-glass contact to preserve the integrity of the product through transportation.
In summary, by offering both nest & tub and tray configurations, Sterinity provides an efficient solution for different fill and finish requirements, with a portfolio of glass vials that answers directly to market needs.
Looking ahead
The growing parenteral market is looking for proven solutions to streamline processing, to focus effort on value added/core activities and to cut production costs, particularly as the structure of the industry changes. With many products now developed and manufactured in collaborative partnership between pharmaceutical companies, biotech start-ups, CROs and CMOs there is a growing need for flexible processing solutions that facilitate safe and reproducible technology transfer. RTU is a cost-effective, efficient approach for aseptic filling, a safety critical but non-core activity for many organizations with molded vials essential for many applications.
The launch of Sterinity, the first commercial solution for RTU molded vials, offers significant potential to reduce the costs associated with aseptic filling while at the same time improving flexibility, quality and safety. By offering the highest quality molded vials for all types of drugs this new product extends the multiple benefits of RTU to a wider range of applications, supporting the industry in bringing products to market more quickly and efficiently.
References:
[1] IQVIA MIDAS, Actual 2018 – Global market database covering 93 countries and over four million pharmaceutical packs.
[2] FDA Guidance for Industry: Sterile Drug Products Produced By Aseptic Processing - Current Good Manufacturing Practice, September 2004
[3] Revision of Annex 1 "Manufacture of Sterile Medicinal Products", December 2017
[4] USP 40 Physical Tests /<660> Containers – Glass. Available to view at: https://hmc.usp.org/sites/default/files/documents/HMC/GCs-Pdfs/c660.pdf
[5] ISO 11135:2014 Sterilization of health -care products – Ethylene Oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices.



































