L’engagement innovation de SGD Pharma
 GET INFUSED PAR SGD PHARMA
GET INFUSED PAR SGD PHARMA
Si SGD Pharma a pu établir sa position de leader dans le secteur du verre, c'est principalement en raison de son aspiration à innover continuellement. En travaillant main dans la main avec ses clients et en étant sans cesse à l'affût des dernières technologies, SGD Pharma tente de résoudre les défis propres à son secteur grâce à la recherche ou l'application de nouvelles solutions technologiques. En substance, SGD Pharma connecte ses produits, ses processus et ses services afin de fournir une solution d'emballage unique et intégrée et répondre aux besoins en constante évolution de ses clients. SGD Pharma s'efforce aussi bien de résoudre le problème d'emballage spécifique d'un client que de développer de nouvelles innovations pour le marché mondial du verre.
SGD Pharma suit l'évolution du marché du verre et reste à l'écoute de ses clients afin d'anticiper leurs besoins actuels et de trouver des solutions à leurs défis. Nos collaborateurs, qui travaillent ensemble au sein d'équipes polyvalentes telles que la R&D, les ventes et le marketing, la Qualité, les affaires réglementaires, l'environnement, Santé et sécurité et les opérations, sont le moteur de notre innovation. Nous veillons à ce que les innovations soient d'une part techniquement viables, et d'autre part, à ce qu'elles répondent aux exigences qualitatives et réglementaires de nos clients.
SGD Pharma œuvre aujourd'hui à relever les défis de demain.
Un processus clair
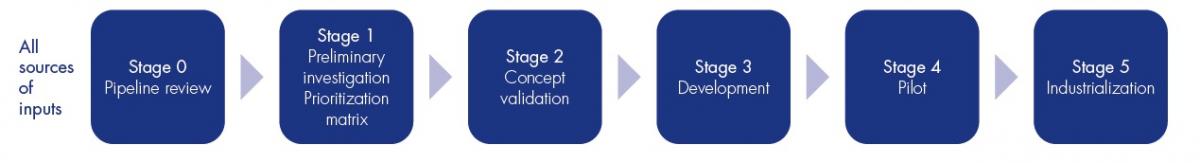
Des innovations adoptées par nos clients
| DES PRODUITS ET DES SERVICES INNOVANTS SGD Pharma a toujours dédié des ressources importantes en recherche et développement. Ces efforts continus nous ont permis d’être :
SGD Pharma a plusieurs succès à son actif :
|